Intermolecular Forces
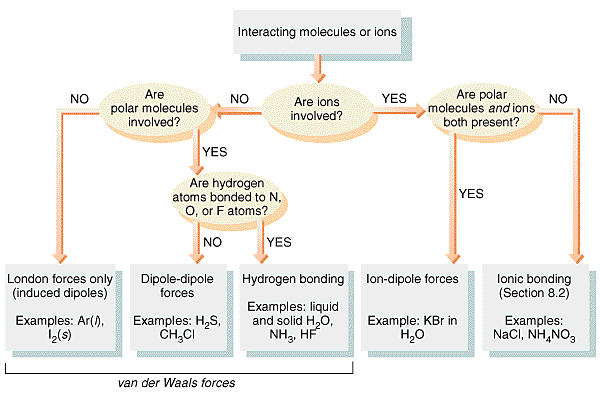
Intermolecular forces(IMFs) are forces of attraction or repulsion which act between neighboring particles (atoms, molecules, or ions). They are weak compared to the intramolecular forces, the forces which keep a molecule together. For example the covalent bond, involving the sharing of electron pairs between atoms is much stronger than the forces present between the neighboring molecules.
There are three main types of intermolecular forces that exist between particle:
London or Dispersion Forces (LF)
London interactions are caused by random fluctuations of electron density in an electron cloud. An atom with a large number of electrons will have a greater associated London force than an atom with fewer electrons.
The London dispersion force is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms form temporary dipoles. This force is sometimes called an induced dipole-induced dipole attraction.
Dispersion forces are present between all molecules, whether they are polar or nonpolar, with larger and heavier atoms and molecules exhibit stronger dispersion forces than smaller and lighter ones.The shapes of molecules also affect the magnitudes of dispersion forces between them.

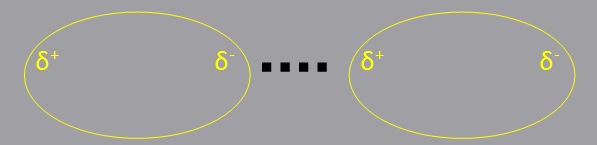
Dipole-Dipole Attractions (DD)
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule.
Polar molecules have a partial negative end and a partial positive end. The partially positive end of a polar molecule is attracted to the partially negative end of another.

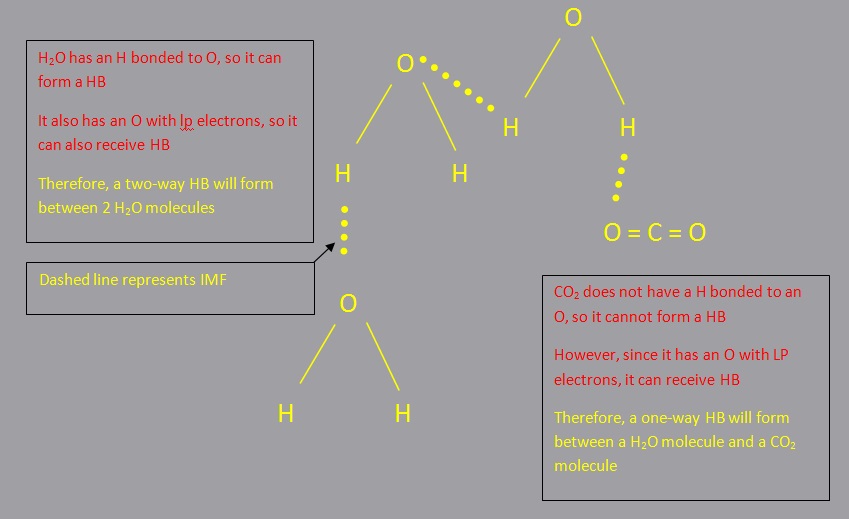
Hydrogen Bonding (HB)
A hydrogen bond is the attraction between a hydrogen atom that is bonded to either nitrogen, oxygen, or fluorine and the lone pair electrons on a nitrogen, oxygen, or fluorine atom of an adjacent molecule.
This attraction or hydrogen bond" can have about 5% to 10% of the strength of a covalent bond.
A HB can be classified as with a two-way HB or one-way HB.
Determing the IMF present in a molecule