| Click to Run |
Discovery of subatomic particles: Rutherford and the Proton
Simulation:
Rutherford Scattering
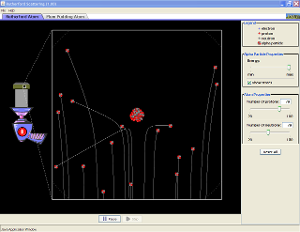
A demonstration of the Rutherford Gold Foil Experiment showing the results that would have validated the Plum Pudding Model and also the results which show the Plum Pudding Model to not be valid.
Ernest Rutherford had established that alpha (α) particles are positively charged particles. They are emitted at high kinetic energies by some radioactive atoms, that is, atoms that disintegrate spontaneously. In 1910, Rutherford’s research group carried out a series of experiments that had an enormous impact on the scientific world. They bombarded a very thin piece of gold foil with α-particles from a radioactive source. A fluorescent zinc sulfide screen was placed behind the foil to indicate the scattering of the α-particles by the gold foil. Scintillations (flashes) on the screen, caused by the individual α -particles, were counted to determine the relative numbers of α-particles deflected at various angles. Alpha particles were known to be extremely dense, much denser than gold.
If the Thomson model of the atom were correct, any α-particles passing through the foil would have been deflected by very small angles. Quite unexpectedly, nearly all of the α-particles passed through the foil with little or no deflection. A few, however, were deflected through large angles, and a very few α-particles even returned from the gold foil in the direction from which they had come!
He suggested that each atom contains a tiny, positively charged, massive center that he called an atomic nucleus. Most α –particles pass through metal foils undeflected because atoms are primarily empty space populated only by the very light electrons, which were around the atomic nucleus. The few particles that are deflected are the ones that come close to the heavy, highly charged metal nuclei (Figure 5-5).
Rutherford was able to determine the magnitudes of the positive charges on the atomic nuclei. The picture of atomic structure that he developed is called the Rutherford model of the atom or Nuclear Model.