General rules for drawing Lewis structure
The following is a list of rules that can be used to determine the Lewis structure of a molecule:
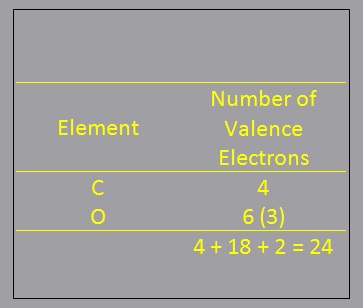
- Determine the number of valence electrons present: Count up the total number of valence electrons. First add up the group numbers of all atoms in the molecule. If the molecule is an anion, add one electron for each unit of charge on the anion. If it is a cation, subtract one electron for each unit of charge on the cation
- Determine the total required number of electrons: Calculate the total number of electrons that would be needed for each atom to have an octet (or doublet for H)
- Determine the number of bonds: Subtract the result of step 1 (number of electrons your have) from the result of step 2 (number of electrons required). This is the total number of shared or bonding electrons.
- Determine the number of electrons remaining and found in lone pairs: Assign two bonding electrons to each bond
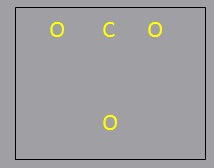
- Generate a template structure for the molecule. Place the peripheral atoms around each other, with the central atom (the atoms with highest valence, the largest atoms, or
the least electronegative atom)
- Filling Octet: Assign remaining electrons as lone pairs, giving octets to all atoms except H
- Filling the central atoms Valence Energy Level: If bonding electrons remain, assign them in pairs making some of the bonds double or triple bonds. (Usually, only C,N,O, and S can form double bonds, and only C and N can form triple bonds). There may be more than one way to do this. Keep all possible structures that result
- Determine the formal charges and put them next to the appropriate atoms. (A formal charge of 0 need not be written explicitly). Check that the formal charges add up to the total charge on the molecule/ion. Do this for all structures obtained in step 5. The structure with the smallest formal charges should be considered as the preferred structure.
Example: Determine the Lewis Structure for CO32-
- Determine the number of valence electrons present :
- Determine the total required number of electrons:
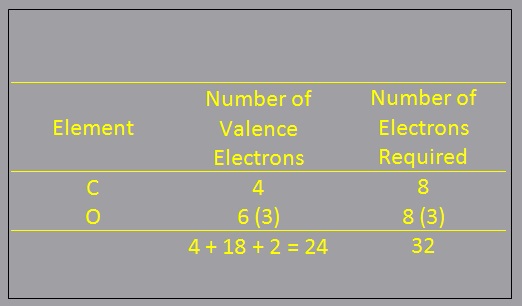
- Determine the number of bonds:

- Determine the number of electrons remaining and found in lone pairs:

- Generate a template structure for the molecule.
- Filling Octet:


- Filling the central atoms Valence Energy Level:
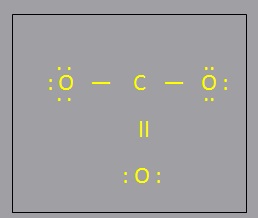
- Determine the formal charges and put them next to the appropriate atoms.


















