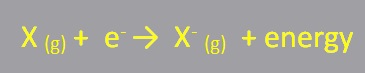Periodic Trends - Effective Nuclear Charge and Force of Attraction
Many properties of elements have a particular pattern or peridicity within the Periodic Table, resulting from the force of attraction that exists between an atomsnucleus and its valence electrons. This force of attraction is proportional to to factors:
- effective nuclear charge
- distance from the nucleus to the valence electrons
| Force of attraction α |
Effective Nuclear Charge |
| Distance |
The effective nuclear charge is the net positive charge experienced by valence electrons. It results from the attractive pull of the protons to the nucleus and the respulsion from the inner core electrons. The core electrons provide a shielding effect, which decreases the attraction that electrons feel for the protons in the nucleus.
- The effective nuclear charge is the net positive charge experienced by valence electrons. It can be approximated by the equation: Zeff = Z - S, where Z is the atomic number and S is the number of shielding electrons (core electrons).
Example:
| Element |
Atomic number |
Core Electrons |
ENC (Zeff) |
| Na |
11 |
10 |
1+ |
| O |
8 |
2 |
6+ |
| Sr |
38 |
36 |
2+ |
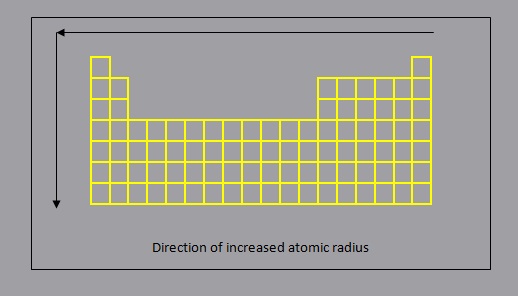
Atomic Radius
The atomic radius of a chemical element is a measure of the size of its atoms, usually the mean or typical distance from the nucleus to the boundary of the surrounding cloud of electrons.
Three widely used definitions of atomic radius are Van der Waals radius, ionic radius, and covalent radius. Widely used definitions of atomic radius include:
- Van der Waals radius: in principle, half the minimum distance between the nuclei of two atoms of the element that are not bound to the same molecule.
- Ionic radius: the nominal radius of the ions of an element in a specific ionization state, deduced from the spacing of atomic nuclei in crystalline salts that include that ion.
- In principle, the spacing between two adjacent oppositely charged ions (the length of the ionic bond between them) should equal the sum of their ionic radii
- Covalent radius: the nominal radius of the atoms of an element when covalently bound to other atoms, as deduced from the separation between the atomic nuclei in molecules.
- In principle, the distance between two atoms that are bound to each other in a molecule (the length of that covalent bond) should equal the sum of their covalent radii.
In general:
- atomic radius increases down a group, as each additional period (row) represents the addition of an energy level which is further from the nucleus
- atomic radius decreases across a period, from left to right as a result of increased force of attraction due to increasing ENC
The following table summarizes the main phenomena that influence the atomic radius of an element:
factor |
principle |
increase with... |
tend to |
effect on radius |
electron shells |
quantum mechanics |
principal quantum numbers |
increase atomic radius |
increases down each column |
effective nuclear charge |
attractive force acting on electrons by protons in nucleus |
atomic number |
decrease atomic radius |
decreases along each period |
shielding |
repulsive force acting on outermost shell electrons by inner electrons |
number of electron shells |
increase atomic radius |
reduces the effect of the 2nd factor |
Ionic Radii
The following guidelines are often considered in the order given.
- Simple positively charged ions (cations) are always smaller than the neutral atoms from which they are formed.
- Simple negatively charged ions (anions) are always larger than the neutral atoms from which they are formed.
- The sizes of cations decrease from left to right across a period.
- The sizes of anions decrease from left to right across a period.
- Within an isoelectronic series, radii decrease with increasing atomic number because of increasing nuclear charge.
- Both cation and anion sizes increase going down a group.
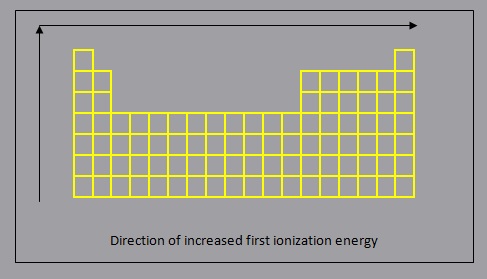
Ionization Energy
The ionization energy of an atom or molecule describes the amount of energy required to remove an electron from the atom or molecule in the gaseous state.

Ionization energy is typically specified as a molar quantity (molar ionization energy or enthalpy) and is reported in units of kJ/mol or kcal/mol (the energy needed to remove one mole of electrons from one mole of atoms or molecules).
In general:
- first ionization energy decreases down a group, as each additional period (row) represents the addition of an energy level which is further from the nucleus, resulting in a lower force of attraction between the nucleus and the electrons in the valence energy level, making it easier to remove an electron
- first ionization energy increases across a period, from left to right, as a result of increased force of attraction due to increasing ENC which will result in a greater amount of energy required to remove an electron from the valence energy level
Electron Affinity
In chemistry and atomic physics, the electron affinity of an atom or molecule is defined as the amount of energy released when an electron is added to a neutral atom or molecule to form a negative ion.
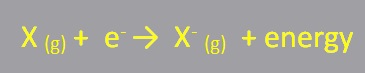
In general:
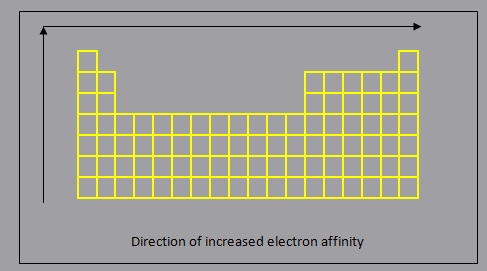
- electron affinity decreases down a group, as each additional period (row) represents the addition of an energy level which is further from the nucleus, resulting in a lower force of attraction between the nucleus and the electrons in the valence energy level, making it harder to gain an electron
- electron affinity increases across a period, from left to right, as a result of increased force of attraction due to increasing ENC which will result in a greater amount of energy released when an electron is added to the valence energy level
- non-metals have a greater electron affinity, release more energy, than metals
- atoms whose anions are more stable than neutral atoms have a greater electron affinity
- the electron affinities of the noble gases have not been conclusively measured, so they may or may not have slightly negative values
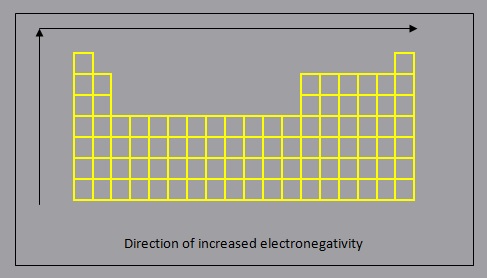
Electronegativity
Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons.
In general:
- electronegativity decreases down a group, as each additional period (row) represents the addition of an energy level which is further from the nucleus, resulting in a lower force of attraction between the nucleus and the bonding pair electrons
- electronegativity increases across a period, from left to right, as a result of increased force of attraction due to increasing ENC which will result in a greater to the bonding pair electrons