Version A
- A student titrates a 35.00 mL sample of a 0.150 mol/L weak acid (Ka = 4.500 x 10-5) with a strong base (0.100 mol/L) solution.
- Calculate the ph at each of the intervals (show all your work):
- Initial pH
- Final pH
- Volume for equivalency
- pH at equivalency
- pH at ½ equivalency
- Sketch the curve for the titration. Label each of the above points on your curve.
- Determine the pH of a buffered hydrofluoric acid solution (HF) if a student adds 2.560 g of HF and 1.850 g of BaF2 to 2.000 L of water (6.3 x 10-4).
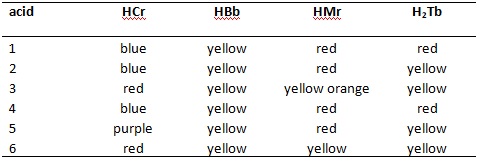
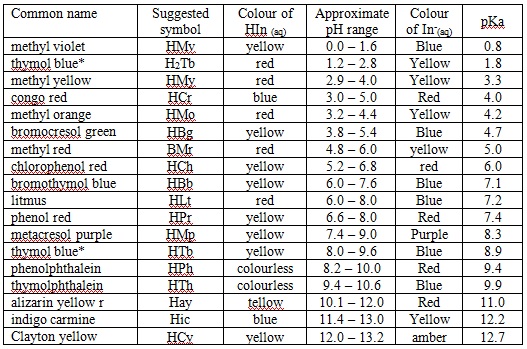
- To determine the various strengths of a series of acids a scientist prepares a 0.01 mol / L solution of each of them. They then divide the sample into 4 parts and add one of the following indicators to each of the indicators. Using the information they gathered in the table below, rank each of the acids in terms of their ionization constant strength, be sure to show ALL of the work to justify your answer.