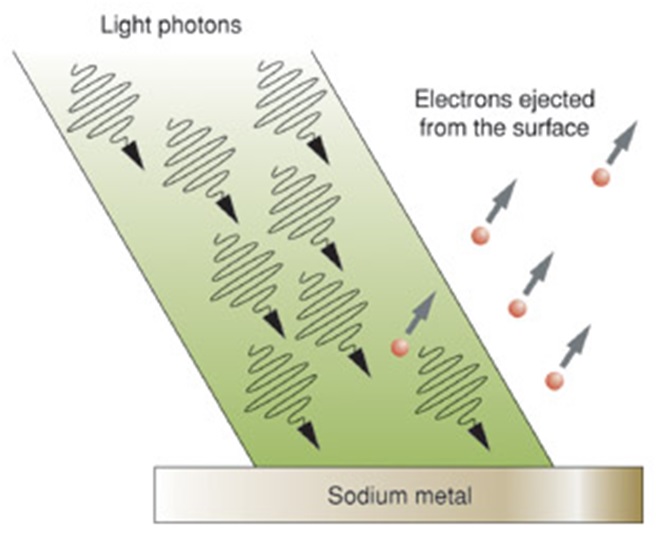
Photoelectric Effect
| In the photoelectric effect, electrons are emitted from matter (metals and non-metallic solids, liquids or gases) as a consequence of their absorption of energy from electromagnetic radiation of very short wavelength, such as visible or ultraviolet light. Electrons emitted in this manner may be referred to as "photoelectrons". First observed by Heinrich Hertz in 1887, the phenomenon is also known as the "Hertz effect", although the latter term has fallen out of general use. Hertz observed and then showed that electrodes illuminated with ultraviolet light create electric sparks more easily. |  |
The photoelectric effect requires photons with energies from a few electronvolts to over 1 MeV in high atomic number elements. Study of the photoelectric effect led to important steps in understanding the quantum nature of light and electrons and influenced the formation of the concept of wave–particle duality.
Emission mechanism
The photons of a light beam have a characteristic energy determined by the frequency of the light. In the photoemission process, if an electron within some material absorbs the energy of a photon has sufficient energy, than the electron is ejected. If the photon energy is too low, the electron is unable to escape the material. Increasing the intensity of the light beam increases the number of photons in the light beam, and thus increases the number of electrons excited, but does not increase the energy that each electron possesses. The energy of the emitted electrons does not depend on the intensity of the incoming light, but only on the energy or frequency of the individual photons. It is an interaction between the incident photon and the outermost electron.
In 1905, Albert Einstein solved this apparent paradox by describing light as composed of discrete quanta, now called photons, rather than continuous waves. Based upon Max Planck's theory of black-body radiation, Einstein theorized that the energy in each quantum of light was equal to the frequency multiplied by a constant, later called Planck's constant. A photon above a threshold frequency has the required energy to eject a single electron, creating the observed effect. This discovery led to the quantum revolution in physics and earned Einstein the Nobel Prize in Physics in 1921. By wave-particle duality the effect can be analyzed purely in terms of waves though not as conveniently.
Simulation
http://phet.colorado.edu/en/simulation/photoelectric
A black body is an idealized physical body that absorbs all incident electromagnetic radiation. Because of this perfect absorptivity at all wavelengths, a black body is also the best possible emitter of thermal radiation, which it radiates incandescently in a characteristic, continuous spectrum that depends on the body's temperature. At Earth-ambient temperatures this emission is in the infrared region of the electromagnetic spectrum and is not visible. The object appears black, since it does not reflect or emit any visible light.
The thermal radiation from a black body is energy converted electrodynamically from the body's pool of internal thermal energy at any temperature greater than absolute zero. It is called blackbody radiation and has a frequency distribution with a characteristic frequency of maximum radiative power that shifts to higher frequencies with increasing temperature. As the temperature increases past a few hundred degrees Celsius, black bodies start to emit visible wavelengths, appearing red, orange, yellow, white, and blue with increasing temperature. When an object is visually white, it is emitting a substantial fraction as ultraviolet radiation.
All matter emits electromagnetic radiation when it has a temperature above absolute zero. The radiation represents a conversion of a body's thermal energy into electromagnetic energy, and is therefore called thermal radiation. It is a spontaneous process of radiative distribution of entropy.
Conversely all matter absorbs electromagnetic radiation to some degree. An object that absorbs all radiation falling on it, at all wavelengths, is called a black body. When a black body is at a uniform temperature, its emission has a characteristic frequency distribution that depends on the temperature. Its emission is called blackbody radiation.
In 1894 Planck turned his attention to the problem of black-body radiation.
The central assumption behind his Planck’s explanation of Black-body radiation, was the supposition, now known as the Planck postulate, that electromagnetic energy could be emitted only in quantized form, in other words, the energy could only be a multiple of an elementary unit E = hν, where h is Planck's constant, also known as Planck's action quantum (introduced already in 1899), and ν (the Greek letter nu, not the Roman letter v) is the frequency of the radiation.
Physicists now call these quanta photons (an elementary particle, the quantum of the electromagnetic interaction and the basic unit of light and all other forms of electromagnetic radiation.).
Simulation: