bears the hydroxyl group.
bears the hydroxyl group. Alcohol
In chemistry, an alcohol is any organic compound in which a hydroxyl functional group (-OH) is bound to a carbon atom, usually connected to other carbon or hydrogen atoms.
Alcohols are classified into primary, secondary and tertiary, based upon the number of carbon atoms connected to the carbon atom that bears the hydroxyl group.
bears the hydroxyl group.
Nomenclature
The suffix -ol appears in the IUPAC chemical name of all substances where the hydroxyl group is the functional group with the highest priority; in substances where a higher priority group is present the prefix hydroxy- will appear in the IUPAC name.
In the IUPAC system, the name of the alkane chain loses the terminal "e" and adds "ol", e.g. "methanol" and "ethanol". When necessary, the position of the hydroxyl group is indicated by a number between the alkane name and the "ol":
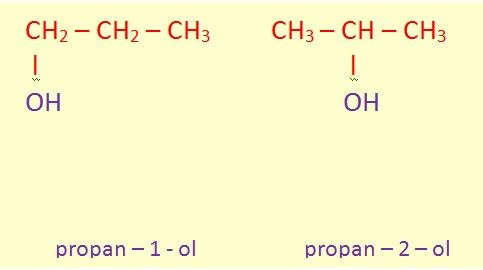
Sometimes, the position number is written before the IUPAC name: 1-propanol and 2-propanol.
Physical and chemical properties
Alcohols have an odor that is often described as “biting” and as “hanging” in the nasal passages.
The hydroxyl group generally makes the alcohol molecule polar. Those groups can form hydrogen bonds to one another and to other compounds (except in certain large molecules where the hydroxyl is protected by steric hindrance of adjacent groups). This hydrogen bonding means that alcohols can be used as protic solvents.
Two opposing solubility trends in alcohols are: the tendency of the polar OH to promote solubility in water, and of the carbon chain to resist it. Thus, methanol, ethanol, and propanol are miscible in water because the hydroxyl group wins out over the short carbon chain. Butanol, with a four-carbon chain, is moderately soluble because of a balance between the two trends. Alcohols of five or more carbons (Pentanol and higher) are effectively insoluble in water because of the hydrocarbon chain's dominance. All simple alcohols are miscible in organic solvents.
Because of hydrogen bonding, alcohols tend to have higher boiling points than comparable hydrocarbons and ethers..
Alcohols, like water, can show either acidic or basic properties at the O-H group. They are generally slightly weaker acids than water, but they are still able to react with strong bases such as sodium hydride or reactive metals such as sodium.
Alcohol PreparationSeveral methods exist for the preparation of alcohols in the laboratory.
SubstitutionPrimary alkyl halides react with aqueous NaOH or KOH mainly to primary alcohols in nucleophilic aliphatic substitution. (Secondary and especially tertiary alkyl halides will give the elimination (alkene) product instead).

Aldehydes or ketones are reduced to form either primary or secondary alcohols.
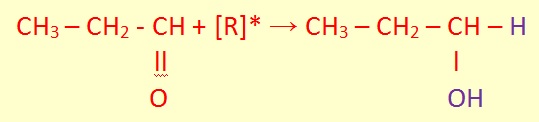

Alkenes engage in an acid catalysed hydration reaction using concentrated sulfuric acid as a catalyst which gives usually secondary or tertiary alcohols. The formation of a secondary alcohol via reduction and hydration is shown:
Alcohol Reactions
DeprotonationAlcohols can behave as weak acids, undergoing deprotonation. The deprotonation reaction to produce an alkoxide salt. The acidity of alcohols is also affected by the overall stability of the alkoxide ion.
Nucleophilic substitutionThe OH group is not a good leaving group in nucleophilic substitution reactions, so neutral alcohols do not react in such reactions. However, if the oxygen is first protonated to give R−OH2+, the leaving group (water) is much more stable, and the nucleophilic substitution can take place.
DehydrationAlcohols are themselves nucleophilic, so R−OH2+ can react with ROH to produce ethers and water in a dehydration reaction, although this reaction is rarely used except in the manufacture of diethyl ether

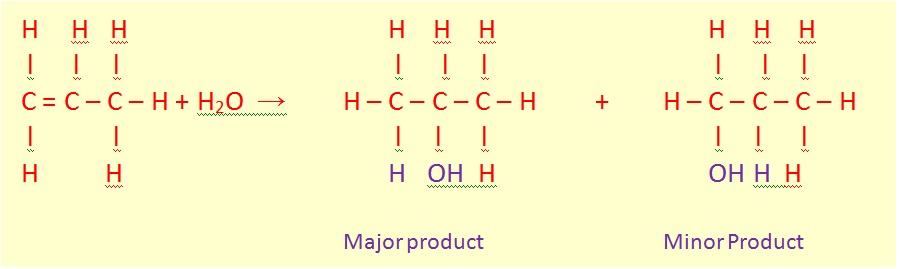
More useful is the E1 elimination reaction of alcohols to produce alkenes. The reaction generally obeys Zaitsev's Rule, which states that the most stable (usually the most substituted) alkene is formed. Tertiary alcohols eliminate easily at just above room temperature, but primary alcohols require a higher temperature.
This is a diagram of acid catalysed dehydration of ethanol to produce ethene:

To form an ester from an alcohol and a carboxylic acid the reaction, known as Fischer esterification, is usually performed at reflux with a catalyst of concentrated sulfuric acid:

In order to drive the equilibrium to the right and produce a good yield of ester, water is usually removed, either by an excess of H2SO4 or by using a Dean-Stark apparatus.
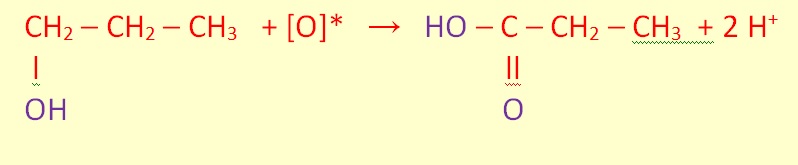
Primary alcohols can be oxidized either to aldehydes or to carboxylic acids.
The oxidation of secondary alcohols normally terminates at the ketone stage.
Tertiary alcohols are resistant to oxidation.
The direct oxidation of primary alcohols to carboxylic acids normally proceeds via the corresponding aldehyde, which is transformed via an aldehyde hydrate (R-CH(OH)2) by reaction with water before it can be further oxidized to the carboxylic acid.