
Alkene
In organic chemistry, an alkene is an unsaturated chemical compound containing at least one carbon-to-carbon double bond. The simplest acyclic alkenes, with only one double bond and no other functional groups, form an homologous series of hydrocarbons with the general formula CnH2n.
Physical propertiesThe physical properties of alkenes are comparable with those of alkanes. The physical state depends on molecular mass (gases from ethene to butene - liquids from pentene onwards).
Boiling Points
The boiling point of each alkene is very similar to that of the alkane with the same number of carbon atoms. Ethene, propene and the various butenes are gases at room temperature. All the rest that you are likely to come across are liquids.
In each case, the alkene has a boiling point which is a small number of degrees lower than the corresponding alkane. The only attractions involved are Van der Waals dispersion forces, and these depend on the shape of the molecule and the number of electrons it contains. Each alkene has 2 fewer electrons than the alkane with the same number of carbons.
Solubility
Alkenes are virtually insoluble in water, but dissolve in organic solvents.
Chemical properties
Alkenes are relatively stable compounds, but are more reactive than alkanes due to the presence of a carbon-carbon double bond It is also attributed to the presence of pi-electrons in the molecule. The majority of the reactions of alkenes involve the rupture of this double bond (pi bond), forming new single bonds.
Alkenes serve as a feedstock for the petrochemical industry because they can participate in a wide variety of reactions.
Alkenes react in many addition reactions, which occur by opening up the double-bond. Most addition reactions to alkenes follow the mechanism of electrophilic addition

If you start from an unsymmetrical alkene like propene, you have to be careful to think about how the addition occurs across the carbon-carbon double bond is the entity being added has the general formula HX. This addition will result in more than one possible prodict. To determine which product will be produced in greater quatities, we use Markovnikov's Rule.
Markovnikov's Rule says that when you add a molecule HX across a carbon-carbon double bond, the hydrogen joins to the carbon atom which already has the more hydrogen atoms attached to it.
Hydrogenation
Hydrogenation of alkenes produces the corresponding alkanes.
The reaction is carried out under pressure at a temperature of 200oC in the presence of a metallic catalyst. Common industrial catalysts are based on platinum, nickel or palladium. For laboratory syntheses, Raney nickel (an alloy of nickel and aluminium) is often employed.

Hydration reaction
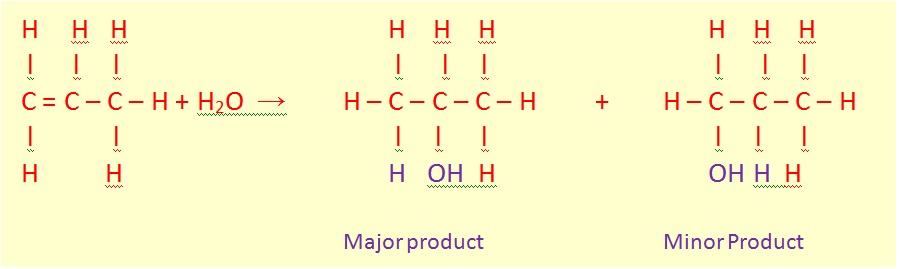
Hydration reaction is a chemical reaction in which a hydroxyl group (OH-) and a hydrogen an acidic proton are added to the two carbon atoms bonded together in the carbon-carbon double bond which makes up an alkene functional group. The reaction usually runs in a strong acidic, aqueous solution.
The general chemical equation of the reaction is the following:

If you start from an unsymmetrical alkene like propene, you have to be careful to think about which way around the water adds across the carbon-carbon double bond.
Markovnikov's Rule says that when you add a molecule HX across a carbon-carbon double bond, the hydrogen joins to the carbon atom which already has the more hydrogen atoms attached to it.
Thinking of water as H-OH, the hydrogen will add to the carbon with the more hydrogens already attached. That means that in the propene case, you will get propan-2-ol rather than propan-1-ol.
Halogenation
In electrophilic halogenation the addition of elemental bromine or chlorine to alkenes yields vicinal dibromo- and dichloroalkanes, respectively. The decoloration of a solution of bromine in water is an analytical test for the presence of alkenes:

Hydrohalogenation is the addition of hydrohalic acids such as HCl or HBr to alkenes to yield the corresponding haloalkanes.

If the two carbon atoms at the double bond are linked to a different number of hydrogen atoms, the halogen is found preferentially at the carbon with fewer hydrogen substituents (Markovnikov's rule).
Oxidation
Alkenes are oxidized with a large number of oxidizing agents.

In the specific case of disubstituted alkenes where the two carbons have one substituent each, Cis-trans notation may be used. If both substituents are on the same side of the bond, it is defined as (cis-). If the substituents are on either side of the bond, it is defined as (trans-).
