Amines
Amines are organic compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, wherein one or more hydrogen atoms have been replace d by a substituent such as an alkyl group
 |
 |
 |
||
| primary amine | secondary amine | tertiary amine |
Nomenclature and Structure of Amines
The nomenclature of amines is complicated by the fact that several different nomenclature systems exist, and there is no clear preference for one over the others. Furthermore, the terms primary (1o), secondary (2o) & tertiary (3o) are used to classify amines in a completely different manner than they were used for alcohols or alkyl halides.
Amines are named for the attached alkane chain with the suffix "-amine". If necessary, the bonding position is infixed.
 |
3 - pentanamine |
For secondary amines (of the form R-NH-R), the longest carbon chain attached to the nitrogen atom becomes the primary name of the amine; the other chain is prefixed as an alkyl group with location prefix given as an italic N.
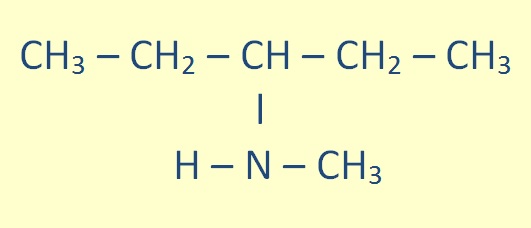 |
N-methylpentan-2-amine |
Tertiary amines (R-NR-R) are treated similarly:
 |
N - ethyl - N - methylpentan-2- amine |
Again, the substituent groups are ordered alphabetically.
Physical propertiesHydrogen bonding significantly influences the properties of primary and secondary amines. Thus the boiling point of an amine is higher than those of the corresponding alkane, but generally lower than those of the corresponding alcohol. For example, methylamine and ethylamine are gases under standard conditions, whereas the corresponding methyl alcohol and ethyl alcohols are liquids.
Gaseous amines possess a characteristic ammonia smell, liquid amines have a distinctive "fishy" smell.
Also reflecting their ability to form hydrogen bonds, most aliphatic amines display some solubility in water. Solubility decreases with the increase in the number of carbon atoms.
Aliphatic amines display significant solubility in organic solvents, especially polar organic solvents.
The aromatic amines, such as aniline, have their lone pair electrons conjugated into the benzene ring, thus their tendency to engage in hydrogen bonding is diminished. Their boiling points are high and their solubility in water low
It is instructive to compare the boiling points and water solubility of amines with those of corresponding alcohols and ethers. The dominant factor here is hydrogen bonding, and the first table below documents the powerful intermolecular attraction that results from -O-H---O- hydrogen bonding in alcohols. Corresponding -N-H---N- hydrogen bonding is weaker, as the lower boiling points of similarly sized amines demonstrate. Alkanes provide reference compounds in which hydrogen bonding is not possible, and the increase in boiling point for equivalent 1º-amines is roughly half the increase observed for equivalent alcohols.
| Compound | CH3CH3 | CH3OH | CH3NH2 | CH3CH2CH3 | CH3OH | CH3CH2NH2 |
| Mol Mass | 30 | 32 | 31 | 44 | 46 | 45 |
| Bp | -88.6 | 62 | -6 | -42 | 78.5 | 19.6 |
The water solubility of 1o and 2o-amines is similar to that of comparable alcohols. As expected, the water solubility of 3o-amines and ethers is also similar. These comparisons, however, are valid only for pure compounds in neutral water. The basicity of amines allows them to be dissolved in dilute mineral acid solutions, and this property facilitates their separation from neutral compounds such as alcohols and hydrocarbons by partitioning between the phases of non-miscible solvents
Basicity of Amines
Like ammonia, most amines are Brønsted and Lewis bases, but their base strength can be changed enormously by substituents.
Amine Reactions
Amine Formation
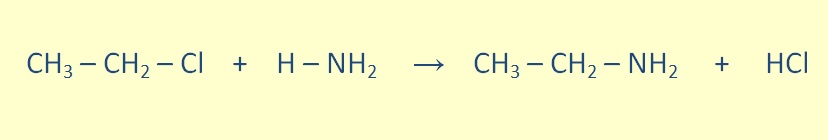
Primary amines can be formed through the reaction of an alkyl halide with ammonia.
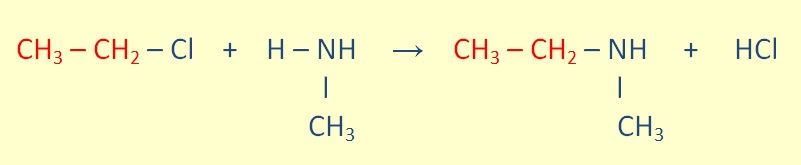
Seconday amines can be formed throught The reaction of a primary amine with an alkyl halide.
Tertiary amines can be formed through he reaction of a secondary amine with an alkyl halide.

Aminde Formation
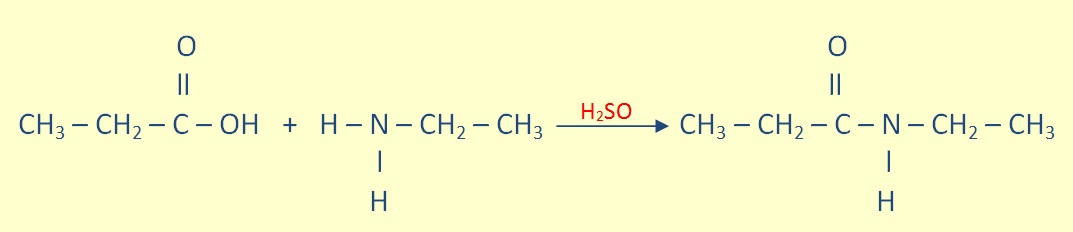
Primary and secondary amines can undergo a condensation reaction with carboxylic acid to form an amide.