
Carboxylic acids are organic acids characterized by the presence of at least one carboxyl group. The general formula of a carboxylic acid is R-COOH, where R is some monovalent functional group. A carboxyl group (or carboxy) is a functional group consisting of a carbonyl and a hydroxyl, which has the formula -C(=O)OH, usually written as -COOH or -CO2H.

Carboxylic acids are Brønsted-Lowry acids, they are proton donors. They are the most common type of organic acid. Among the simplest examples are the formic acid HCOOH, that occurs in ants, and acetic acid CH3COOH group, that gives vinegar its sour taste. Acids with two or more carboxyl groups are called dicarboxylic, tricarboxylic, etc. The simplest dicarboxylic example is oxalic acid (COOH)2, which is just two connected carboxyls. Other important natural examples are citric acid (in lemons) and tartaric acid (in tamarinds).
The parent chain must include the carboxyl carbon, which is given position number 1. The name of the alkane attached is changed by replacing the -e with -oic acid.
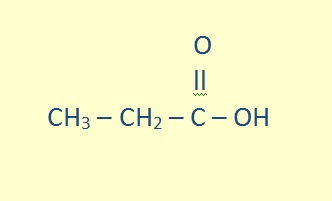 |
propanoic acid |
Physical properties
The polar nature of both the O-H and C=O bonds (due to the electonegativity difference of the atoms) results in the formation of strong hydrogen bonds with other carboxylic acid molecules or other H-bonding systems
SolubilityCarboxylic acids are polar. Because they are both hydrogen-bond acceptors (the carbonyl) and hydrogen-bond donors (the hydroxyl), they also participate in hydrogen bonding. Together the hydroxyl and carbonyl group forms the functional group carboxyl.
Carboxylic acids usually exist as dimeric pairs in nonpolar media due to their tendency to “self-associate.”
Smaller carboxylic acids (1 to 5 carbons) are soluble with water, whereas higher carboxylic acids are less soluble due to the increasing hydrophobic nature of the alkyl chain.
These longer chain acids tend to be rather soluble in less-polar solvents such as ethers and alcohols.
Boiling pointsCarboxylic acids tend to have higher boiling points than water, not only because of their increased surface area, but because of their tendency to form stabilised dimers. Carboxylic acids tend to evaporate or boil as these dimers. For boiling to occur, either the dimer bonds must be broken, or the entire dimer arrangement must be vaporised, both of which increase enthalpy of vaporisation requirements significantly.
AcidityCarboxylic acids are typically weak acids, meaning that they only partially dissociate into H+ cations and RCOO– anions in neutral aqueous solution. For example, at room temperature, only 0.02 % of all acetic acid molecules are dissociated. Electronegative substituents give stronger acids.
OdorCarboxylic acids often have strong odors, especially the volatile derivatives. Most common are acetic acid (vinegar) and butyric acid (rancid butter). On the other hand, esters of carboxylic acids tend to have pleasant odors and many are used in perfumes
Carboxylic Acid Reactions
Carboxylic acids are used to synthesize two important derivatives, esters and amides.
Esterification
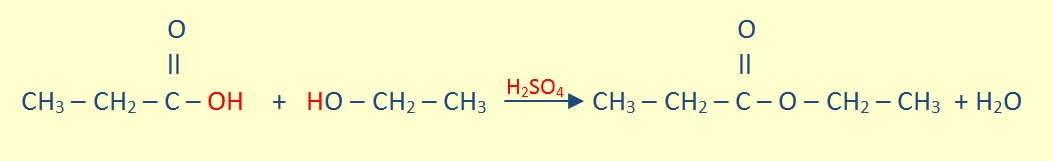
In an mixture with carboxylic alcohol, an equilibrium reaction forms ester and water in the presence of heat and a strong acid catalyst.
For example:
Amides of Carboxylic Acids
In amides, the OH of the carboxyl group is replaced by nitrogen holding any combination of H atoms or hydrocarbon groups.

The preparation of simple amides are similar to those of esters, but they are in an excess of ammonia.
When amides are in water, they are changed back to the carboxylic acid and ammonia. Unlike amines, the amides are nonbasic even though they have the NH2 group in simple amides. This is because of the O atom in the carbonyl group, which is very electronegative. This tightens the electrons on N so that it is unable to accept a proton.