In the reaction between calcium carbonate and dilute hydrochloric acid
HCl + calcium carbonate → calcium chloride + carbon dioxide + water.
HCl(aq)+ CaCO3 (s)→ CaCl2 (aq) + CO2 (g) + H2O (l)
calcium carbonate may be used in the form of marble chips.

The reaction rates can be compared using large marble chips, and the same mass of small marble chips.
The reaction can be followed by plotting the loss of mass against time.
The reaction rate is faster (the slope is steeper) for the reaction with small marble chips (greater surface area).
Note that the final loss of mass is the same for both reactions. This is because the same mass of calcium carbonate (marble chips) will give the same mass of carbon dioxide whether the chips are large or small. The smaller chips will just do it more quickly.

Therefore, collision theory explains the affect of increasing surface area on the rate of a reaction by:
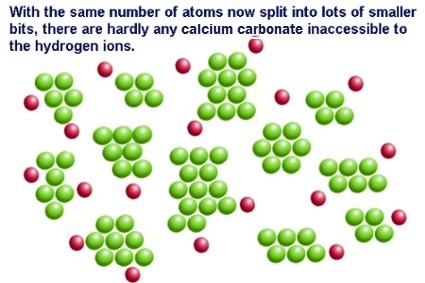
- at higher surface area there are more particles exposed to a potential reaction, this increased exposure will allow for an increase in the number of collision that can occur
Applilet:
http://www.absorblearning.com/chemistry/demo/units/LR1502.html#Increasingsurfacearea