Galvanic Cells
Galvanic Cells or Voltaic Cells consist of a reduction reaction and an oxidation reaction which occurs in separate electrolytes, with the two electrolytes connect through a salt bridge and two electrodes joined by a wire. The electric current is the result of the spontaneous redox reaction that occurs. We measure the potential of the cell.
A salt bridge serves three functions.
1. It allows electrical contact between the two solutions.
2. It prevents mixing of the electrode solutions.
3. It maintains the electrical neutrality in each half-cell as ions flow into and out of the salt bridge.
Example: The Zinc-Copper Cell
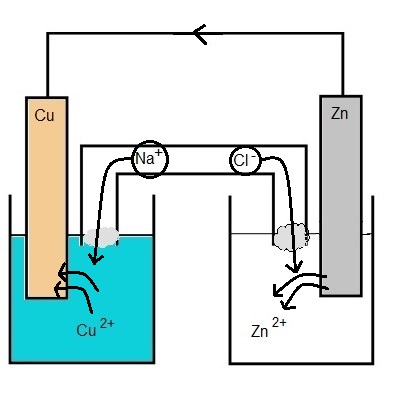
A piece of zinc was placed into a beaker containing ZnCl2 and a piece of copper was placed in a beaker containing CuCl2 (aq). The two beakers are connected with a salt bridge that has NaBr (aq) in it.
- Sketch the galvanic cell
- Determine the overall redox reaction
- Determine the overall cell potential
- Write the standard notation for the cell
In one beaker there is:
Cu(s), Cu2+ (aq), and Cl-(aq)
and in the other has:
Zn(s), Zn2+ (aq), and Cl-(aq)
In looking the Reduction table, the SOA is Cu2+ and the SRA is Zn (s)
Therefore, the reduction rxn will be:
Cu2+ (aq) + 2 e- → Cu (s) Eoreduction = 0.34 V
And the oxidation rxn will be:
Zn (s) → Zn2+ (aq) + 2 e- Eooxidation = 0.76 V |
 |
Since an oxidation rxn will occur on the Zn (s) electrode, there will be Zn2+ ions added to the soln, leaving behind the lost e-.
This makes the electrode have a negative charge, and the beaker would end up with a net positive charge due to the addition of Zn2+ to the soln. To prevent this, anions travel through the salt bridge into the beaker with the ZnCl2 soln. As a result the Zn (s) electrode is called the anode.
Since a reduction rxn will occur on the Cu (s) electrode, Cu2+ ions are removed from the soln.
This makes the electrode have a positive charge, and the beaker would end up with a net negative charge due to the removal of Cu2+ to the soln. To prevent this, cations travel through the salt bridge into the beaker with the CuCl2 soln. As a result the Zn (s) electrode is called the cathode. |
 |
Electrons travel from the anode through the wire / external circuit to the cathode.
Can combine the two half rxn’s, but have to make sure that the number of electrons are equal btw the 2 half rxns.
Cu2+ (aq) + Zn (s) → Cu (s) + Zn2+ (aq)
Can also determine the overall cell potential using
Eocell = Eoreduction + Eooxidation = 0.34 V + 0.76 V = 1.10 V. |
 |
Standard Notation
Voltaic cells can be represented as follows:
cathode l cathode electrolyte ll anode electrolyte l anode
so for the zinc-copper voltaic cell:
Cu (s) l Cu2+ (aq) ll Zn2+ (aq) l Zn (s) |
Example: The Inert Electrode
A piece of platinum was placed into a beaker containing KMnO4 and HCl and a piece of copper was placed in a beaker containing CuCl2 (aq). The two beakers are connected with a salt bridge that has NaBr (aq) in it.
Sketch the galvanic cell
1. Determine the overall redox reaction
2. Determine the overall cell potential
3. Write the standard notation for the cell
In one beaker there is:
Pt (s), MnO4- (aq), H+ (aq), K+ (aq), and Cl-(aq)
and in the other has:
Cu(s), Cu2+ (aq), and Cl-(aq)
In looking the Reduction table, the SOA is MnO4- / H+ and the SRA is Cu (s)
Therefore, the reduction rxn will be:
MnO4- (aq) + 8 H+ (aq) + 5 e- → Mn2+ (aq) + 4 H2O (l)
Eoreduction = 1.51 V
And the oxidation rxn will be:
Cu (s) → Cu2+ (aq) + 2 e-
Eooxidation = - 0.34 V |
 |
Since an oxidation rxn will occur on the Cu (s) electrode, there will be Cu2+ ions added to the soln, leaving behind the lost e-.
This makes the electrode have a negative charge, and the beaker would end up with a net positive charge due to the addition of Cu2+ to the soln. To prevent this, anions travel through the salt bridge into the beaker with the CuCl2 soln. As a result the Cu (s) electrode is called the anode.
Since a reduction rxn will occur on the Pt (s) electrode, MnO4- ions are redeuced to Mn2+.
This makes the electrode have a positive charge, and the beaker would end up with a net negative charge due to the removal of H+ to the soln. To prevent this, cations travel through the salt bridge into the beaker with the KMNO4 / HCl soln. As a result the Pt (s) electrode is called the cathode. |
 |
| Electrons travel from the anode through the wire / external circuit to the cathode. The sodium ion and chloride ions move into the appropriate electorlytes to ensure solutions do not gain a charge. |
 |
Can combine the two half rxn’s, but have to make sure that the number of electrons are equal btw the 2 half rxns.
2 MnO4- (aq) + 16 H+ (aq) + 5 Cu (s) → 2 Mn2+ (aq) + Cu2+ (aq) + 8 H2O (l)
Can also determine the overall cell potential using
Eocell = Eoreduction + Eooxidation = 1.51 V + 0.34 V = 1.85 V. |





