Electrolytic Cells
Oxidation-reduction or redox reactions take place in electrochemical cells. There are two types of electrochemical cells. Spontaneous reactions occur in galvanic (voltaic) cells; nonspontaneous reactions occur in electrolytic cells. Both types of cells contain electrodes where the oxidation and reduction reactions occur. Oxidation occurs at the electrode termed the anode and reduction occurs at the electrode called the cathode.
Electrodes & Charge
The anode of an electrolytic cell is positive (cathode is negative), since the anode attracts anions from the solution. However, the anode of a galvanic cell is negatively charged, since the spontaneous oxidation at the anode is the source of the cell's electrons or negative charge. The cathode of a galvanic cell is its positive terminal. In both galvanic and electrolytic cells, oxidation takes place at the anode and electrons flow from the anode to the cathode
Electrolytic Cells
The redox reaction in an electrolytic cell is nonspontaneous. Electrical energy is required to induce the electrolysis reaction. An example of an electrolytic cell is shown below, in which molten NaCl is electrolyzed to form liquid sodium and chlorine gas. The sodium ions migrate toward the cathode, where they are reduced to sodium metal. Similarly, chloride ions migrate to the anode and are oxided to form chlorine gas. This type of cell is used to produce sodium and chlorine. The chlorine gas can be collected surrounding the cell. The sodium metal is less dense than the molten salt and is removed as it floats to the top of the reaction container.
Example: A Electrolytic Cell in molten NaCl
Two inert electrodes are placed into molten NaCl.
- Sketch the electrolytic cell
- Determine the overall redox reaction
- Determine the overall cell potential
| The electrode connected to the negative end of the power source will have electrons flow into it and become negatively charged. |
 |
The electrode connected to the positive end of the power source will draw electrons up out of the electrode causing it to have it positive charge. |
The negatively charged electrode will attract the positive sodium ions.
As a result, it is called the cathode.
Since the cathode has an excess of electrons, it will pass them to the Na+ ions, causing a reduction reaction.
Na+ (l) + e- → Na (l)
The potential required to reduce Na+ is -2.71 V |
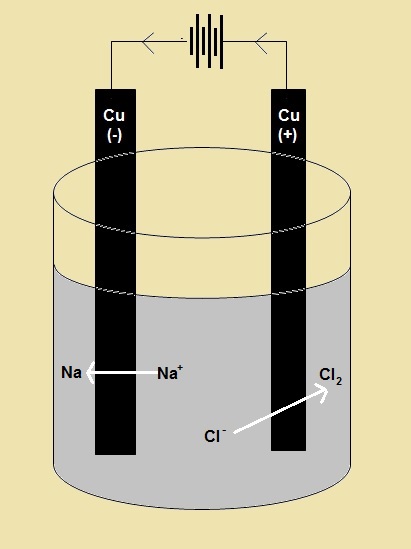 |
The positively charged electrode will negative chloride ions.
As a result, it is called the anode.
Since the anode is losing electrons, it will look to get them back, and will take them from the Cl- ions, causing an oxidation reaction.
2 Cl- (l) → Cl2 (g) + 2e-
The potential for the reduction of chlorine is - 1.36 V. |
Overall reaction:
2 Cl- + 2 Na+ → Na (l) + Cl2 (g)
To determine the overall cell potential: Eo = Eoreduction + Eooxidation = -2.71 + – 1.36 = -4.07
Therefore for the cell to cause the reduction reaction below it has to be connected to a power source with a potential of 4.07 V. |
Example: The Potassium Iodide Cell
Two inert electrodes are placed into KI (aq)
- Determine the overall redox reaction
- Determine the overall cell potential
- Sketch the electrolytic cell
First determine what entitiest are present in the solution.
Second, identify them as oxidizing agen and reducing agent
| RA |
|
|
| I-(aq) |
H2O (l) |
K+ (aq) |
| |
OA |
OA |
Third, Identify the strongest oxidizing and reducing agents.
| SRA |
|
|
| I-(aq) |
H2O (l) |
K+ (aq) |
| |
SOA |
OA |
Therefore:
the oxidation reaction that will occur at the anode is:
2 I- (aq) → I2 (s) + 2 e- Eooxidation = - 0.54 V
the reduction reaction that will occur at the cathode is:
2 H2O (l) + 2 e- → H2 (g) + 2 OH- (aq) Eoreduction = - 0.83 V
|
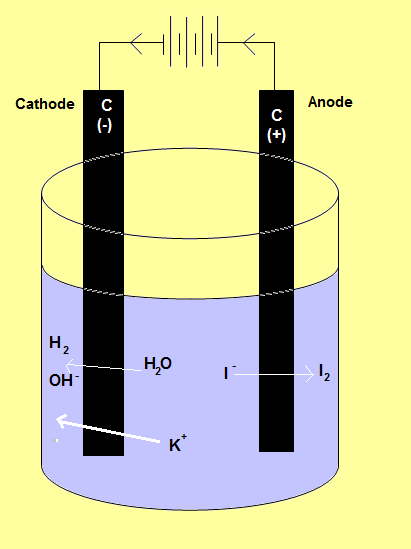 |
| The overall redox reaction will be:
2 I- (aq) + 2 H2O (l) + 2 e- → I2 (s) + H2 (g) + 2 OH- (aq)
To determine the overall cell potential: Eo = Eoreduction+ Eooxidation = -2.71 + – 1.36 = - 4.07
|
Comparison between Galvanic and Electrolytic Cells
|
Galvanic Cell |
Electrolytic Cell |
Spontaneity |
spontaneous reaction |
non-spontaneous reaction |
Standard Cell Potential (ΔEo) |
positive |
negative |
Cathode |
strongest oxidizing agent present
undergoes a reduction
positive electrode |
strongest oxidizing agent present
undergoes a reduction
negative electrode |
Anode |
strongest reducing agent present
undergoes an oxidation
negative electrode |
strongest reducing agent present
undergoes an oxidation
positive electrode |
Direction of electron flow |
anode → cathode |
anode → cathode |
Direction of ion movement |
anions → anode
cations → cathode |
anions → anode
cations → cathode |


